Research on Sickle Cell Anaemie
“A tiny change today brings a dramatically different tomorrow.”
— Richard Bach
Sickle Cell Disease (SCD) is a life-threatening, inherited blood disorder that significantly shortens life expectancy and severely impairs quality of life. It is especially widespread in sub-Saharan Africa, where healthcare systems are often ill-equipped to manage the disease. Existing treatment options are scarce, costly, and offer only limited clinical benefit. In most cases, care is restricted to basic symptom management, which fails to prevent serious complications or provide sustainable health improvements.
In response to this urgent need, the treatment PC1434n was introduced in 2013 in several African countries. A 2018 study concluded that PC1434n offers a cost-effective, accessible alternative, showing clear improvements in pain reduction, overall health, and functioning across age groups (See graph). ARHF calls for broader application, training, and research to expand its reach to underserved populations.
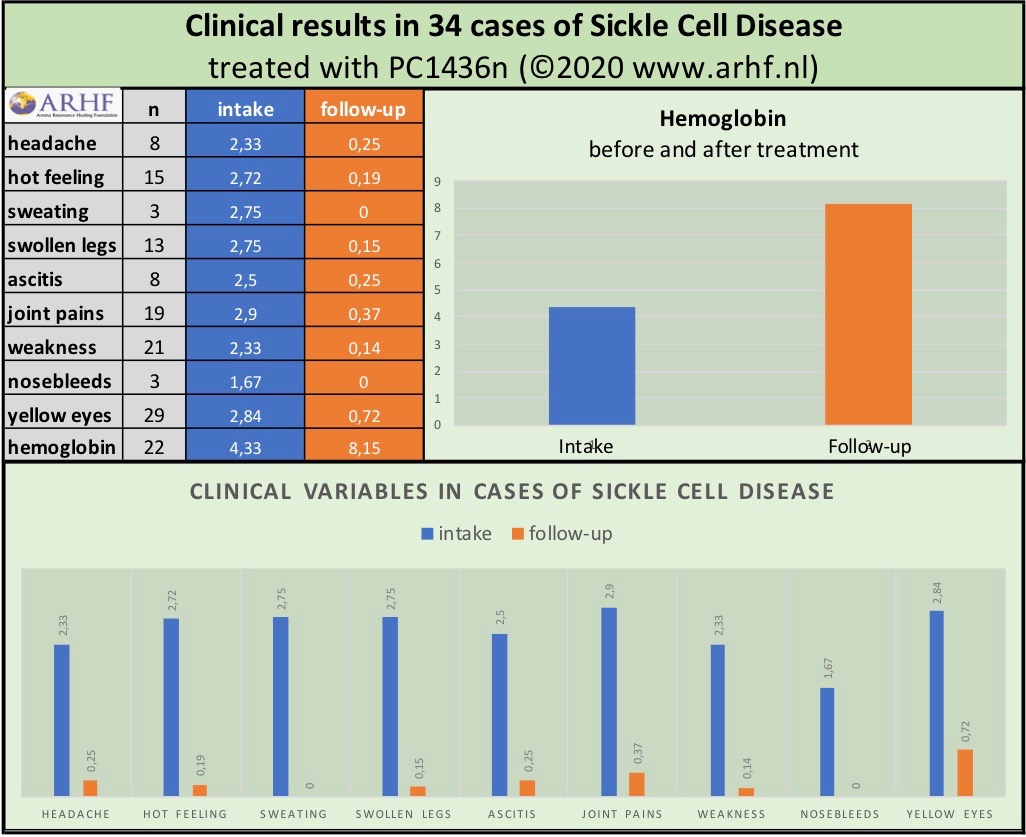
Sickle cell disease continues to cause intense pain, fatigue, and life-threatening complications for millions. ARHF’s natural, information-based approach offers a promising complementary path for relief and improved quality of life. Your support can help advance this important research and reach those in need.
Exploring New Avenues in Sickle Cell Support and Symptom Relief
Upcoming Study by ARHF

References
- Achieng B, Omondi M, Balikwisha N, Hongo Y, Akpabio E, Chappell P, Van der Zee H. Treating Sickle Cell Disease in Africa. Homoeopathic Links 2018;31(2):120–126






